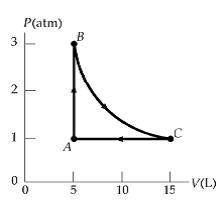
II. Practice An ideal gas occupies 5 L at atmospheric pressure and 300 K (point A). It is warmed at constant volume to 3 atm (point B). Then it is allowed to expand isothermally to 1 atm (point C) and at last compressed isobarically to its original state. A. How many moles of gas are being used? B. Find the temperature at point C. C. Find the work done on the gas in each process. D. Find the amount of heat added to/removed from the gas in one cycle.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1



Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
II. Practice An ideal gas occupies 5 L at atmospheric pressure and 300 K (point A). It is warmed at...
Questions


Mathematics, 21.06.2020 21:57


Physics, 21.06.2020 21:57


Mathematics, 21.06.2020 21:57



Mathematics, 21.06.2020 21:57


English, 21.06.2020 21:57


Chemistry, 21.06.2020 21:57



Mathematics, 21.06.2020 21:57

Chemistry, 21.06.2020 21:57

Mathematics, 21.06.2020 21:57

Mathematics, 21.06.2020 21:57

Mathematics, 21.06.2020 21:57