
Consider the two reduction half-reactions: Na+(aq) + e− LaTeX: \longrightarrow⟶ Na(s) Eo = −2.71 V Cl2(g) + 2 e− LaTeX: \longrightarrow⟶ 2 Cl−(aq) Eo = 1.36 VUse the electrode potentials above to calculate Eocell and ∆Gorxn for the reaction below, and determine if it is the reaction for a voltaic cell or an electrolytic cell. 2 Na+(aq) + 2 Cl−(aq) LaTeX: \longrightarrow⟶2 Na(s) + Cl2(g)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Consider the two reduction half-reactions: Na+(aq) + e− LaTeX: \longrightarrow⟶ Na(s) Eo = −2.71 V C...
Questions




English, 16.10.2020 06:01



Spanish, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01




Mathematics, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01


Mathematics, 16.10.2020 06:01

History, 16.10.2020 06:01




Mathematics, 16.10.2020 06:01

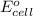 = -4.07v
= -4.07v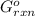 = +785.510 KJ
= +785.510 KJ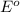 and Δ
and Δ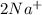 +
+ 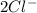 →
→ 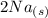 +
+ 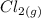
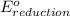 - Δ
- Δ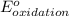 = -2.71v - 1.36v = -4.07V
= -2.71v - 1.36v = -4.07V

