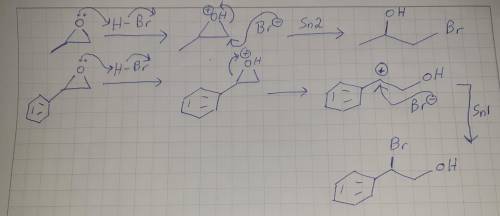
When methyloxirane is treated with HBr, the bromide ion attacks the less substituted position. However, when phenyloxirane is treated with HBr, the bromide ion attacks the more substituted position. Explain the difference in regiochemistry in terms of a competition between steric effects and electronic effects. (Hint: It may help to draw out the structure of the phenyl group.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
When methyloxirane is treated with HBr, the bromide ion attacks the less substituted position. Howev...
Questions



History, 29.09.2019 10:20

Social Studies, 29.09.2019 10:20

Mathematics, 29.09.2019 10:20

Mathematics, 29.09.2019 10:20

English, 29.09.2019 10:20


Biology, 29.09.2019 10:20

Mathematics, 29.09.2019 10:20

History, 29.09.2019 10:20

Biology, 29.09.2019 10:20



Mathematics, 29.09.2019 10:20

History, 29.09.2019 10:20

History, 29.09.2019 10:20



Chemistry, 29.09.2019 10:20