Chemistry, 05.05.2020 04:13 Lizzy527663
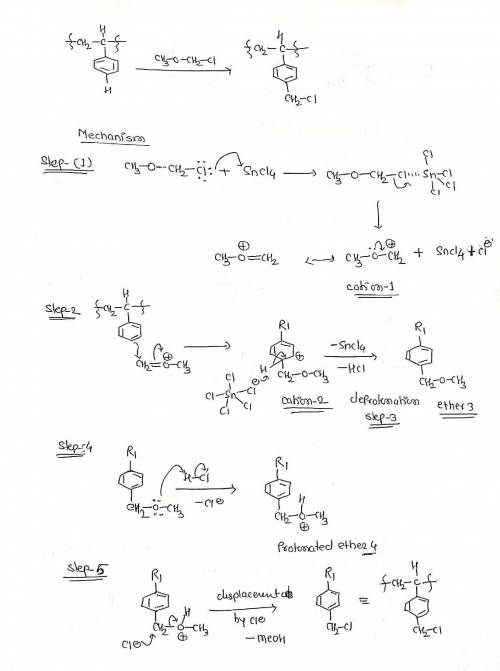
The chloromethylated polystyrene resin used for Merrifield solid-phase peptide synthesis is prepared by treatment of polystyrene with chloromethyl methyl ether and a Lewis acid catalyst. The reaction involves the following steps: Reaction of the ether with the Lewis acid to form cation 1; Electrophilic aromatic substitution to form resonance stabilized cation 2; Deprotonation yields aromatic ether 3; Protonation to form protonated ether 4; Displacement by chloride ion to form the final product. Write out the mechanism on a separate sheet of paper and then draw the structure of the resonance contributors of the resonance stabilized cation 2.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
The chloromethylated polystyrene resin used for Merrifield solid-phase peptide synthesis is prepared...
Questions

Mathematics, 27.10.2021 14:00


Biology, 27.10.2021 14:00



Health, 27.10.2021 14:00

English, 27.10.2021 14:00


Mathematics, 27.10.2021 14:00



Social Studies, 27.10.2021 14:00


Physics, 27.10.2021 14:00

Mathematics, 27.10.2021 14:00

Computers and Technology, 27.10.2021 14:00


English, 27.10.2021 14:00

Biology, 27.10.2021 14:00

Mathematics, 27.10.2021 14:00