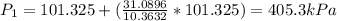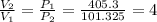Chemistry, 05.05.2020 04:21 smithmalyk4
A scuba diver that ascends to the surface too quickly can experience decompression sickness, which occurs when nitrogen that dissolves in the blood under high pressure, forms bubbles as the pressure decreases during the ascent. Therefore, an understanding of the gas laws is an important part of a scuba diver's training. In fresh water, the pressure increases by 1 atm every 34 ft below the water surface a diver descends.
If a diver ascends quickly to the surface from a depth of 102 ft without exhaling, by what factor will the volume of the diver's lungs change upon arrival at the surface?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1


Chemistry, 23.06.2019 06:30
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1
You know the right answer?
A scuba diver that ascends to the surface too quickly can experience decompression sickness, which o...
Questions


Mathematics, 07.11.2020 07:00

Mathematics, 07.11.2020 07:00


Mathematics, 07.11.2020 07:00




English, 07.11.2020 07:00

Biology, 07.11.2020 07:00

Mathematics, 07.11.2020 07:00

Mathematics, 07.11.2020 07:00


Mathematics, 07.11.2020 07:00



Biology, 07.11.2020 07:00


Social Studies, 07.11.2020 07:00

History, 07.11.2020 07:00