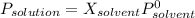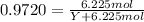Chemistry, 05.05.2020 04:23 NylaJohn29
The common laboratory solvent ethanol is often used to purify substances dissolved in it. The vapor pressure of ethanol , CH3CH2OH, is 54.68 mm Hg at 25 °C. In a laboratory experiment, students synthesized a new compound and found that when 32.83 grams of the compound were dissolved in 286.8 grams of ethanol, the vapor pressure of the solution was 53.15 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight of this compound? ethanol = CH3CH2OH = 46.07 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
The common laboratory solvent ethanol is often used to purify substances dissolved in it. The vapor...
Questions

Biology, 20.11.2020 03:50


Mathematics, 20.11.2020 03:50




History, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50



Geography, 20.11.2020 03:50



Mathematics, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50

Biology, 20.11.2020 03:50

Social Studies, 20.11.2020 03:50