
Chemistry, 05.05.2020 04:36 kelsgoat22
Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. Suppose 2.1 grams of ethane is mixed with 3.68 grams of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction.
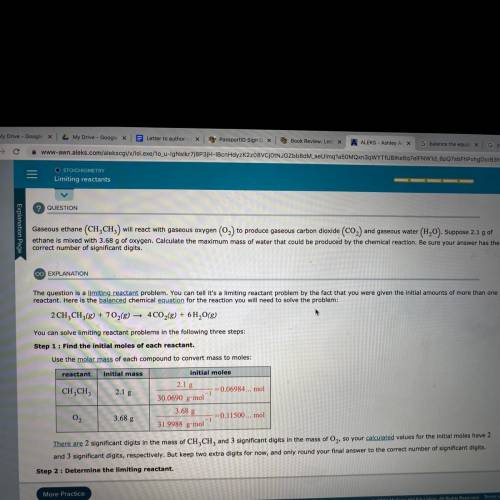

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. S...
Questions

Mathematics, 20.01.2020 23:31


Mathematics, 20.01.2020 23:31


Computers and Technology, 20.01.2020 23:31







Social Studies, 20.01.2020 23:31










