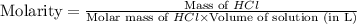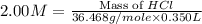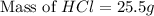Chemistry, 05.05.2020 03:17 aomoloju4202
An aqueous 2.00 M hydrochloric acid solution is prepared with a total volume of 0.350 L. The molecular
weight of hydrochloric acid is 36.468
mol
What mass of hydrochloric acid (in grams) is needed for the solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
An aqueous 2.00 M hydrochloric acid solution is prepared with a total volume of 0.350 L. The molecul...
Questions

Mathematics, 22.08.2020 04:01

History, 22.08.2020 04:01

Physics, 22.08.2020 04:01



Computers and Technology, 22.08.2020 04:01


Social Studies, 22.08.2020 04:01



Mathematics, 22.08.2020 04:01





Geography, 22.08.2020 05:01

English, 22.08.2020 05:01

Mathematics, 22.08.2020 05:01


Biology, 22.08.2020 05:01

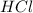 = 2.00 M
= 2.00 M