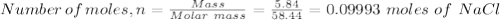A student has a mixture of salt (NaCl) and sugar (C12H22O11). To determine the percentage, the student measures out 5.84 grams of the mixture and dissolves this in 100.0 mL of water. The student knows that aqueous salt with aqueous silver nitrate (AgNO3) to form solid silver chloride (AgCl). The student determines that sugar will not react with silver nitrate. How many mL of 1.0 M AgNO3 would be required to precipitate 5.84 grams of NaCl?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2
You know the right answer?
A student has a mixture of salt (NaCl) and sugar (C12H22O11). To determine the percentage, the stude...
Questions

Mathematics, 20.05.2021 21:40

History, 20.05.2021 21:40


French, 20.05.2021 21:40




History, 20.05.2021 21:40

Mathematics, 20.05.2021 21:40


Computers and Technology, 20.05.2021 21:40



Arts, 20.05.2021 21:40

Mathematics, 20.05.2021 21:40


History, 20.05.2021 21:40

Mathematics, 20.05.2021 21:40


Chemistry, 20.05.2021 21:40