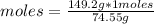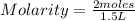Chemistry, 05.05.2020 01:29 Natalierg05
What is the molarity of a solution of KCl if 1500. mL contains 149.2 grams of KCl? (Atomic mass of K = 39.10 g/mol and Cl = 35.45 g/mol)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
What is the molarity of a solution of KCl if 1500. mL contains 149.2 grams of KCl? (Atomic mass of K...
Questions



Physics, 21.02.2020 21:07

Mathematics, 21.02.2020 21:07




Social Studies, 21.02.2020 21:08







English, 21.02.2020 21:08

Mathematics, 21.02.2020 21:08



Mathematics, 21.02.2020 21:09

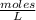
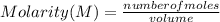
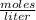 ).
).