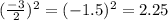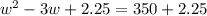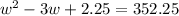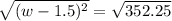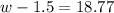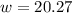Introduction: This activity introduces you to solutions and allows you to experience making different
concentrations of Kool-aid solution. There are many ways to calculate the concentration of a substance
including: molarity (M), parts per million (ppm), percent composition (% comp), and grams per liter (g/L). In
chemistry, concentration is usually measured by the number of moles of substance/ liter of substance, or
Molarity.
Materials:
• Kool-Aid Powder with SUGAR already included (or sugar if you don’t have kool-aid)
• Something to stir solutions
• 4 large cups
• measuring cup (ml preferred but cups will work)
Instructions: In this activity you will be making a series of kool-aid solutions. Make sure to record all data and
answer all the questions below.
Step 1: You will prepare a 237.5 ml kool-aid solution with a concentration of 1M. The molar mass of kool-aid
(or sugar since kool-aid is basically sugar C12H22O11.) is 342 g/mol. Show the calculations below which helped
you create your 1M solution. Note: 237.5 mL is approximately one cup.
Please show your work here.
Liters of water needed
Molarity of kool-aid needed
Grams of kool-aid needed
Now prepare the solution and make sure to label it (sticky note, pen on plastic cup, piece of paper under
cup, etc…)
To make this solution:
1. Measure approximately ½ dry cups of the Kool-aid mixture.
2. Pour the Kool-aid powder you measured into a large glass or cup.
3. Measure out the appropriate amount of water (1 cup) and mix into your glass/cup.
5. Stir to mix and label with the appropriate molarity.
6. Don’t taste test it yet, that will come later.
Do not discard the leftover 1M solution; we are not done with it yet!
Step 2: Using the 1M solution above you will create a second solution with a molarity of 0.5 M solution and
volume of 237.5 mL of kool-aid. Watch video link below for help.
Show your work here. You may need the following equation M1V1=M2V2 where M stands for molarity and V
standards for volume. :
Volume of 1M solution needed
Volume of water needed
Now prepare the solution and make sure to label it! Do not discard the leftover 1M and 0.5 M solutions;
we are not done with them yet!
Step 3: Using the 0.5 M solution above you will create 237.5 mL of a 0.25 M solution of kool-aid. You will need
the following equation M1V1=M2V2 where M stands for molarity and V standards for volume. Note: 237.5 ml is
about 1 cup. There is no video demonstration of this calculation, but follow the same dilution calculation
procedure as was demonstrated in step 2.
Show your work here:
Volume of 0.5 M solution needed:
Volume of water needed:
Now prepare the solution, make sure to label! Do not discard the leftover solutions; we are not done
with them yet!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
Introduction: This activity introduces you to solutions and allows you to experience making differen...
Questions



English, 18.05.2021 02:20






Mathematics, 18.05.2021 02:20




Mathematics, 18.05.2021 02:20






Computers and Technology, 18.05.2021 02:20

History, 18.05.2021 02:20