***NEED HELP***
Plus one more
5. Le Chateliers principle states that when an...

Chemistry, 05.05.2020 00:10 samyajones68
***NEED HELP***
Plus one more
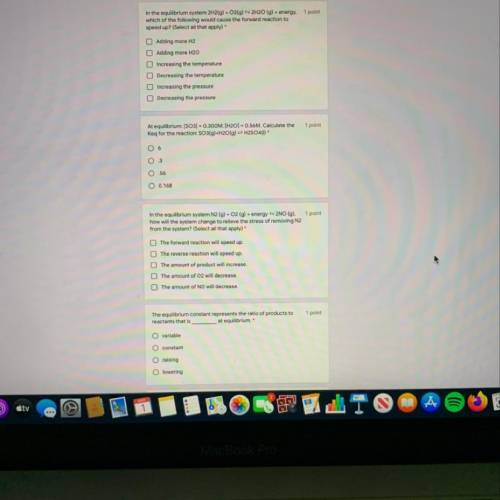
5. Le Chateliers principle states that when an equilibrium system is stressed,
A. The amount of product and reactant will decrease.
B. The amount of product and reactant will increase.
C. The amount of reactants and products will change in such a way so that the stress will be relieved.
D. The forward reaction will speed up.
E. The reverse reaction will increase.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
You know the right answer?
Questions










English, 30.03.2020 17:50




Mathematics, 30.03.2020 17:50


Mathematics, 30.03.2020 17:50



Mathematics, 30.03.2020 17:51



