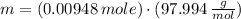Chemistry, 05.05.2020 00:19 gomezjuana123
What is the mass, in grams, of 0.00948\,\text {mol}0.00948mol0, point, 00948, start text, m, o, l, end text of phosphoric acid?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2
You know the right answer?
What is the mass, in grams, of 0.00948\,\text {mol}0.00948mol0, point, 00948, start text, m, o, l, e...
Questions

Physics, 24.11.2019 19:31

Mathematics, 24.11.2019 19:31

Mathematics, 24.11.2019 19:31


Health, 24.11.2019 19:31

Health, 24.11.2019 19:31


Physics, 24.11.2019 19:31

Mathematics, 24.11.2019 19:31

Business, 24.11.2019 19:31


Biology, 24.11.2019 19:31

Mathematics, 24.11.2019 20:31


Mathematics, 24.11.2019 20:31

Mathematics, 24.11.2019 20:31

English, 24.11.2019 20:31



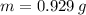
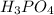 , whose molecular weight is
, whose molecular weight is 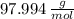 . The mass in grams of the sample is computed afterwards:
. The mass in grams of the sample is computed afterwards: