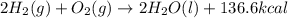Review energy changes by identifying the correct statements about each reaction.
2H2(g) +...

Chemistry, 03.05.2020 13:37 rahmao2773
Review energy changes by identifying the correct statements about each reaction.
2H2(g) + O2(g) ---> 2H2O(l) + 136.6 kcal
ANSWERS:
The reaction is: exothermic
The delta H value is: negative
Heat is a: product

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 23.06.2019 02:00
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
Questions

English, 04.04.2021 20:10



Mathematics, 04.04.2021 20:10

History, 04.04.2021 20:10

English, 04.04.2021 20:10

Mathematics, 04.04.2021 20:10

Mathematics, 04.04.2021 20:20

History, 04.04.2021 20:20




Biology, 04.04.2021 20:20

History, 04.04.2021 20:20

Mathematics, 04.04.2021 20:20

English, 04.04.2021 20:20

Health, 04.04.2021 20:20



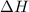 value is negative.
value is negative.