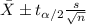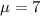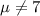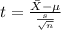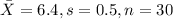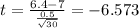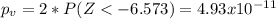Chemistry, 04.05.2020 22:37 lydia1melton
The Environmental Protection Agency has determined that safe drinking
water should have an average pH of 7 moles per liter. You are testing water from a new source, and take 30 vials of water. Water is unsafe if it deviates too far from 7 moles per liter in either direction. The mean pH level in your sample is 6.4 moles per liter, which is slightly acidic. The standard deviation of the sample is 0.5 moles per liter.
b) A 95% confidence interval for the true mean pH level of the water is (6.21, 6.59). Interpret this interval.
c) Explain why the interval in part (b) is consistent with the result of the test in part (a).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3


Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
The Environmental Protection Agency has determined that safe drinking
water should have an ave...
water should have an ave...
Questions


History, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01


History, 14.07.2020 01:01





Mathematics, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Social Studies, 14.07.2020 01:01

English, 14.07.2020 01:01


Computers and Technology, 14.07.2020 01:01