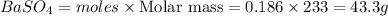Chemistry, 13.01.2020 23:31 bullsfan4584
Calculate the mass of the precipitate formed when 2.27 l of 0.0820 m ba(oh)2 are mixed with 3.06 l of 0.0664 m na2so4.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
Calculate the mass of the precipitate formed when 2.27 l of 0.0820 m ba(oh)2 are mixed with 3.06 l o...
Questions



History, 24.05.2021 21:00



Mathematics, 24.05.2021 21:00



Mathematics, 24.05.2021 21:00


Mathematics, 24.05.2021 21:00


English, 24.05.2021 21:00

Spanish, 24.05.2021 21:00


Mathematics, 24.05.2021 21:00

Mathematics, 24.05.2021 21:00



Health, 24.05.2021 21:00

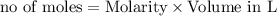
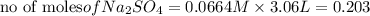
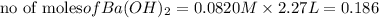
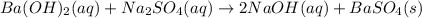
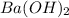 reacts with 1 mole of
reacts with 1 mole of 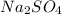
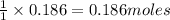 of
of 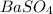 precipitate
precipitate