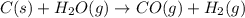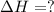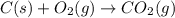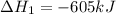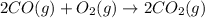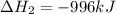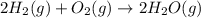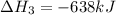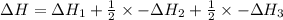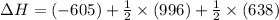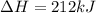Chemistry, 05.05.2020 14:47 clarajeansonels9987
QUESTION 13
Use the standard reaction enthalpies given below to determine AHºrn for the followi
C(s) + H2O(g) - CO(g) + H2(9)
Given:
Reaction 1: C(s) + O2(g) – CO2(g) AHºrxn = -605 kJ
Reaction 2: 2 CO(g) + O2(g) – 2 CO2(g) AH°x = -966 kJ
Reaction 3: 2 H2(g) + O2(g) → 2 H2O(g) AHⓇx = -638 kJ

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
QUESTION 13
Use the standard reaction enthalpies given below to determine AHºrn for the followi...
Use the standard reaction enthalpies given below to determine AHºrn for the followi...
Questions

Mathematics, 26.08.2019 07:30

History, 26.08.2019 07:30

Mathematics, 26.08.2019 07:30


Mathematics, 26.08.2019 07:30


History, 26.08.2019 07:30

English, 26.08.2019 07:30

History, 26.08.2019 07:30




Mathematics, 26.08.2019 07:30

Biology, 26.08.2019 07:30

Chemistry, 26.08.2019 07:30



Chemistry, 26.08.2019 07:30

English, 26.08.2019 07:30

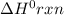 for the reaction is 212 kJ
for the reaction is 212 kJ