Chemistry, 05.05.2020 14:49 hosteenimport21
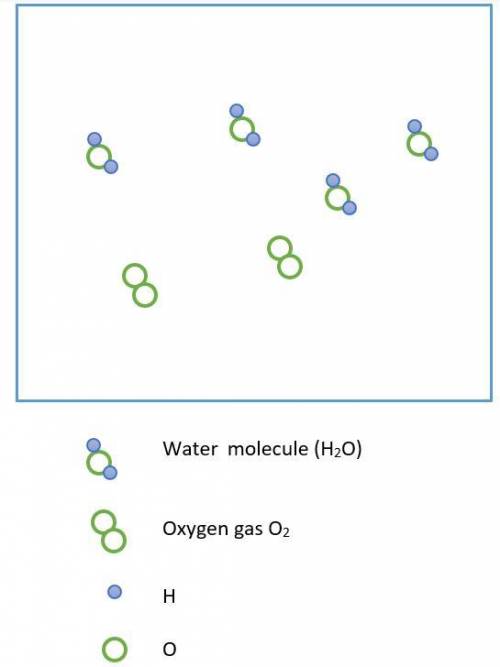
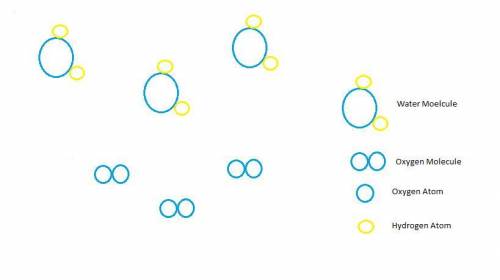
Hydrogen peroxide, H2O2, decomposes according to the equation above. This reaction is thermodynamically favorable at room temperature.
(a) A particulate representation of the reactants is shown below in the box on the left. In the box below on the right, draw the particulate representation of all the molecules that would be produced from these four reactant molecules.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2


Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
Hydrogen peroxide, H2O2, decomposes according to the equation above. This reaction is thermodynamica...
Questions





History, 19.03.2020 03:08





Mathematics, 19.03.2020 03:08

Mathematics, 19.03.2020 03:08


Business, 19.03.2020 03:08

Computers and Technology, 19.03.2020 03:08




Computers and Technology, 19.03.2020 03:09