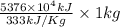On a hot summer day you and some friends decide you want to cool down your pool. Determine the mass of ice you would need to add to bring the equilibrium temperature of the system to 300K. The pool contains 400,000 L (at a density of 1 kg/L) of water initially at 305K. Assume the ice is at 0°C (273K), the heat capacity of water is 4.2 J/(g*K), and the enthalpy of melting ice is 333 kJ/kg.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
On a hot summer day you and some friends decide you want to cool down your pool. Determine the mass...
Questions

Social Studies, 05.11.2020 05:50



Social Studies, 05.11.2020 05:50

History, 05.11.2020 05:50


Mathematics, 05.11.2020 05:50

Advanced Placement (AP), 05.11.2020 05:50

Mathematics, 05.11.2020 05:50

Mathematics, 05.11.2020 05:50


Engineering, 05.11.2020 05:50


History, 05.11.2020 05:50

Mathematics, 05.11.2020 05:50

Chemistry, 05.11.2020 06:00

Spanish, 05.11.2020 06:00

History, 05.11.2020 06:00

Mathematics, 05.11.2020 06:00

History, 05.11.2020 06:00

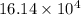 kg.
kg.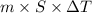

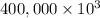 g (as 1 kg = 1000 g)
g (as 1 kg = 1000 g) = 305 K - 273 K
= 305 K - 273 K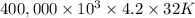
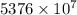 J
J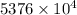 kJ
kJ