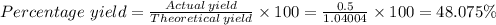100.0mL of 0.40M Al(NO3)3 is reacted with 100.0 mL of 0.40M Al(OH)3 according to
the following reaction: Al(NO3)3 + 3KOH → Al(OH)3 + 3KNO3
1) Identify the limiting reactant.
2) Identify the excess reactant.
3) Determine the theoretical yield of Al(OH)3
4) How much excess reactant is left over after the reaction is complete?
5) If 0.50g of Al(OH)3 determine the percent yield of Al(OH)3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
100.0mL of 0.40M Al(NO3)3 is reacted with 100.0 mL of 0.40M Al(OH)3 according to
the following...
the following...
Questions





Engineering, 05.05.2020 11:56



Mathematics, 05.05.2020 11:56




Mathematics, 05.05.2020 11:56






Mathematics, 05.05.2020 11:57

Mathematics, 05.05.2020 11:57