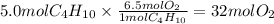Chemistry, 05.05.2020 09:45 michaelmorrison37
Butane, C4H10, is the gas burned in disposable lighters. How many moles of oxygen are needed to burn 5.0 mol of butane in a lighter to produce carbon dioxide and water?
Unbalanced equation:
C4H10 + O2 -> CO2 + H2O
28 mol
32 mol
13 mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1
You know the right answer?
Butane, C4H10, is the gas burned in disposable lighters. How many moles of oxygen are needed to burn...
Questions

Mathematics, 09.02.2021 20:50


Social Studies, 09.02.2021 20:50

Mathematics, 09.02.2021 20:50

Mathematics, 09.02.2021 20:50

Mathematics, 09.02.2021 20:50

Business, 09.02.2021 20:50

Chemistry, 09.02.2021 20:50

Biology, 09.02.2021 20:50


Mathematics, 09.02.2021 20:50






Mathematics, 09.02.2021 20:50

Mathematics, 09.02.2021 20:50


Mathematics, 09.02.2021 20:50