
Chemistry, 05.05.2020 09:46 kezionhoward13
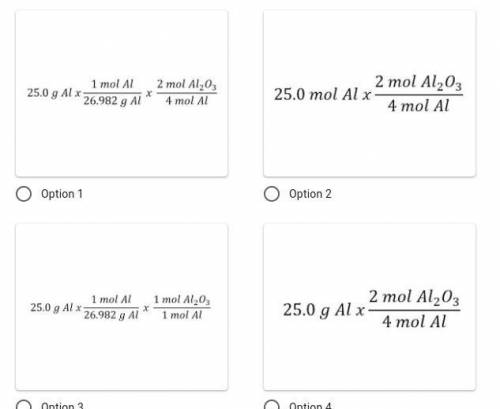
Identify the correct set-up. How many moles of aluminum oxide would form if 25.0 g of aluminum react completely given the following chemical reaction: 4Al + 3O₂ → 2Al₂O₃?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1


Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1
You know the right answer?
Identify the correct set-up. How many moles of aluminum oxide would form if 25.0 g of aluminum react...
Questions

Mathematics, 02.11.2021 03:30

Mathematics, 02.11.2021 03:30


Mathematics, 02.11.2021 03:40

History, 02.11.2021 03:40


Mathematics, 02.11.2021 03:40


History, 02.11.2021 03:40




Chemistry, 02.11.2021 03:40

Advanced Placement (AP), 02.11.2021 03:40

Mathematics, 02.11.2021 03:40





English, 02.11.2021 03:50



