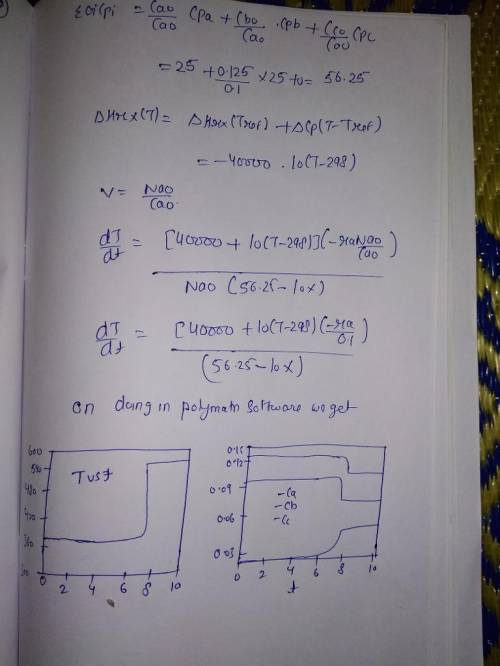
Chemistry, 05.05.2020 16:42 caylah5921
Is carried out adiabatically in a constant-volume batch reactor. The rate law is Plot and analyze the conversion, temperature, and concentrations of the reacting species as a function of time. Additional information: Initial Temperature 100C k1 (373 K) 2 103 s1 E1 100 kJ/mol k2 (373 K) 3 105 s1 E2 150 kJ/mol CA0 0.1 mol/dm3 25 J/mol K CB0 0.125 mol/dm3 25 J/mol K (298 K) 40,000 J/mol A 40 J/mol K

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
Is carried out adiabatically in a constant-volume batch reactor. The rate law is Plot and analyze th...
Questions


Physics, 11.06.2021 22:30



Mathematics, 11.06.2021 22:40

Mathematics, 11.06.2021 22:40






Mathematics, 11.06.2021 22:40


Biology, 11.06.2021 22:40



History, 11.06.2021 22:40


Mathematics, 11.06.2021 22:40