
Chemistry, 05.05.2020 16:44 ladnerhailey16
An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M O2. At this temperature, Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g). What is the equilibrium concentration of CO? An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M O2. At this temperature, Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g). What is the equilibrium concentration of CO? 3.1 × 10-1 M 4.8 × 10-6 M 2.2 × 10-3 M 1.9 × 10 7 M 9.3 × 10-2 M

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3
You know the right answer?
An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M...
Questions

Mathematics, 25.10.2019 10:43



History, 25.10.2019 10:43



History, 25.10.2019 10:43

Mathematics, 25.10.2019 10:43

Mathematics, 25.10.2019 10:43


Social Studies, 25.10.2019 10:43


Social Studies, 25.10.2019 10:43





Mathematics, 25.10.2019 10:43


History, 25.10.2019 10:43

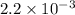 M.
M.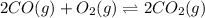
![K_{c}=\frac{[CO_{2}]^{2}}{[CO]^{2}[O_{2}]}](/tpl/images/0640/3657/11981.png)
![[CO_{2}]](/tpl/images/0640/3657/0a7e9.png) ,
, ![[CO]](/tpl/images/0640/3657/32558.png) and
and ![[O_{2}]](/tpl/images/0640/3657/9a638.png) represent equilibrium concentration of
represent equilibrium concentration of 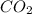 , CO and
, CO and 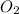 respectively.
respectively.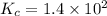
![[CO]=\sqrt{\frac{[CO_{2}]^{2}}{K_{c}.[O_{2}]}}](/tpl/images/0640/3657/02c20.png) M =
M = 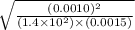 M =
M = 

