Chemistry, 05.05.2020 17:37 canyonmorlan
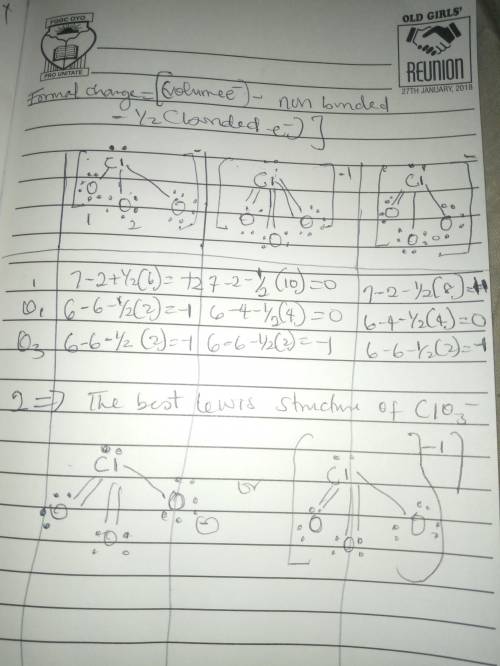
The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms. We can draw three inequivalent Lewis structures for the thiocyanate ion , SCN- . The concepts of formal charge and electronegativity can help us choose the structure that is the best representation.1. Assign formal charges to the elements in each of the structures below. ABCFormal ChargeSCN-12010-100-12. The best Lewis structure for SCN- is .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding e...
Questions

History, 16.07.2019 06:00

Mathematics, 16.07.2019 06:00

Mathematics, 16.07.2019 06:00







Mathematics, 16.07.2019 06:00



English, 16.07.2019 06:00


History, 16.07.2019 06:00

Chemistry, 16.07.2019 06:00

Physics, 16.07.2019 06:00

Mathematics, 16.07.2019 06:00