Chemistry, 05.05.2020 17:45 hilzepesqtatiana
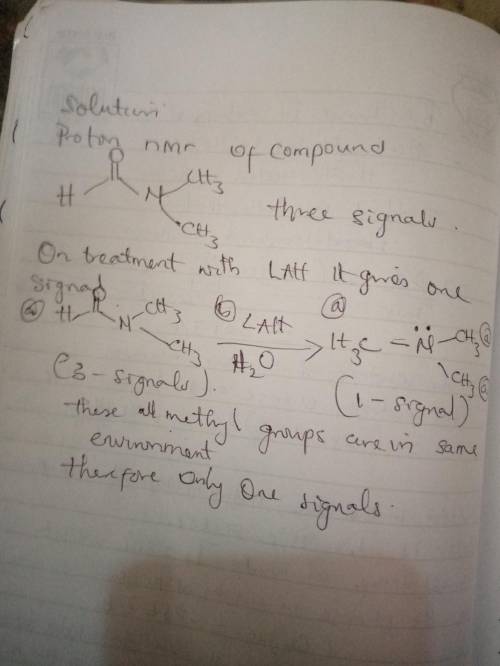
(a) The 1H NMR spectrum of DMF exhibits three signals. Upon treatment with excess LAH followed by water, DMF is converted into a new compound that exhibits only one signal in its 1H NMR spectrum. Explain. DMF, like most , exhibits restricted rotation about the bond between the carbonyl group and the nitrogen atom. This restricted rotation causes the groups to be in different electronic environments. They are not chemically equivalent, and will therefore produce different signals (in addition to the signal from the other proton in the compound). Upon treatment with excess LAH followed by water, DMF is to an amine that exhibit restricted rotation. As such, the methyl groups chemically equivalent and will together produce . (b) Based on your answer to part a, how many signals do you expect in the 13C NMR spectrum of DMF? Restricted rotation causes the methyl groups to be in electronic environments. As a result, the 13C NMR spectrum of DMF should have .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 06:30
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent.true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight.a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
(a) The 1H NMR spectrum of DMF exhibits three signals. Upon treatment with excess LAH followed by wa...
Questions

Mathematics, 17.10.2021 07:40

Mathematics, 17.10.2021 07:40

Biology, 17.10.2021 07:40

Business, 17.10.2021 07:40

History, 17.10.2021 07:40


Biology, 17.10.2021 07:40


Biology, 17.10.2021 07:40


Mathematics, 17.10.2021 07:40



Mathematics, 17.10.2021 07:40


Mathematics, 17.10.2021 07:40

Mathematics, 17.10.2021 07:40