
Chemistry, 05.05.2020 17:44 gapaxton22
The heat capacity of an object is given by the following equation: C equals 14000 straight J over straight K plus open parentheses 200 straight J over straight K squared close parentheses T plus open parentheses 3 straight J over straight K cubed close parentheses T squared What is the change in the entropy of the object (in J/K) associated with raising its temperature from 290 K to 380 K?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
The heat capacity of an object is given by the following equation: C equals 14000 straight J over st...
Questions

History, 21.12.2019 02:31

Mathematics, 21.12.2019 02:31





Mathematics, 21.12.2019 02:31


Health, 21.12.2019 02:31

Mathematics, 21.12.2019 02:31


Mathematics, 21.12.2019 02:31

Mathematics, 21.12.2019 02:31







English, 21.12.2019 02:31

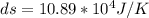
![C = 14000\frac{J}{K} + (200 \frac{J}{K^2} )T + [3 \frac{J}{K^3} ] T^2](/tpl/images/0640/9264/78425.png)
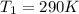
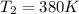
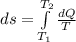
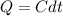
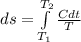
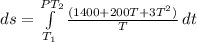
![ds = 1400\ ln [T]+ 200 \frac{T^2}{T} +3 \frac{T^2}{2 T} \ \ | \left \ T_2} \atop {T_1}} \right.](/tpl/images/0640/9264/c257a.png)
![ds = 1400 ln [\frac{T_2}{T_1} ] + 200 (T_2 - T_1 ) + \frac{3}{2} (T_2^2 -T_1^2)](/tpl/images/0640/9264/6b21c.png)
![ds = 1400 ln [\frac{380}{290} ] + 200 (380 - 290 ) + \frac{3}{2} (380^2 -290 ^2)](/tpl/images/0640/9264/2ea63.png)


