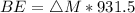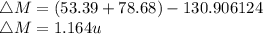Iodine-131 is a radioactive isotope that is used in the treatment of cancer of the thyroid. The natural tendency of the thyroid to take up iodine creates a pathway for which radiation (β− and γ) that is emitted from this unstable nucleus can be directed onto the cancerous tumor with very little collateral damage to surrounding healthy tissue. Another advantage of the isotope is its relatively short half-life (8 days). Calculate the binding energy of iodine-131 and the binding energy per nucleon. The mass of iodine-131 is 130.906124 u.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Which of the following is a testable hypothesis? a. if i brush my teeth, i will get fewer cavities than if i don't brush my teeth. b. green toothpaste tastes better than blue toothpaste or red toothpaste. c. smart, careful, healthy people always brush their teeth. d. it's wrong to not brush your teeth before you have an important conversation with someone.
Answers: 1

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1


Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
Iodine-131 is a radioactive isotope that is used in the treatment of cancer of the thyroid. The natu...
Questions

Mathematics, 24.07.2019 20:10

















Mathematics, 24.07.2019 20:10

English, 24.07.2019 20:10

English, 24.07.2019 20:10