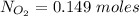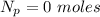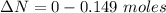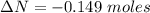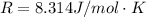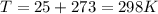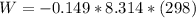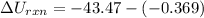The oxidation of copper(I) oxide, Cu2O(s) , to copper(II) oxide, CuO(s) , is an exothermic process. 2Cu2O(s)+O2(g)⟶4CuO(s) The change in enthalpy upon reaction of 42.60 g Cu2O(s) is −43.47 kJ . Calculate the work, , and energy change, Δrxn , when 42.60 g Cu2O(s) is oxidized at a constant pressure of 1.00 bar and a constant temperature of 25∘ C . Note that ΔErxn is sometimes used as the symbol for energy change instead of Δrxn .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
The oxidation of copper(I) oxide, Cu2O(s) , to copper(II) oxide, CuO(s) , is an exothermic process....
Questions


History, 03.02.2022 20:00


Mathematics, 03.02.2022 20:00

Mathematics, 03.02.2022 20:00


Computers and Technology, 03.02.2022 20:00

Mathematics, 03.02.2022 20:00




Mathematics, 03.02.2022 20:00

Mathematics, 03.02.2022 20:00

Mathematics, 03.02.2022 20:00

English, 03.02.2022 20:00

Mathematics, 03.02.2022 20:00

Mathematics, 03.02.2022 20:00

Computers and Technology, 03.02.2022 20:10

Chemistry, 03.02.2022 20:10

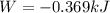
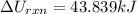

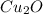 is
is 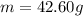
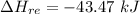 this also the change in energy in terms of heat
this also the change in energy in terms of heat 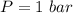
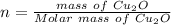
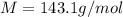
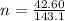
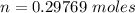
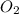 to give Four moles of
to give Four moles of 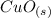
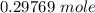 of
of 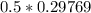 moles of
moles of 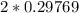 moles of
moles of 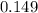 moles of
moles of 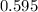 moles of
moles of