
Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of ·0.0038Ms−1: 2NH3(g)→N2(g)+3H2(g) Suppose a 450.mL flask is charged under these conditions with 150.mmol of ammonia. How much is left 20.s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


You know the right answer?
Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of...
Questions




History, 29.06.2019 16:00

English, 29.06.2019 16:00


Mathematics, 29.06.2019 16:00


Social Studies, 29.06.2019 16:00








Physics, 29.06.2019 16:00

Physics, 29.06.2019 16:00

Mathematics, 29.06.2019 16:00

World Languages, 29.06.2019 16:00

 mmol of
mmol of 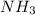 is left after 20 s.
is left after 20 s.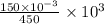 M = 0.333 M
M = 0.333 M![[NH_{3}]=-kt+[NH_{3}]_{0}](/tpl/images/0641/7445/9cd63.png)
![[NH_{3}]](/tpl/images/0641/7445/acd38.png) represents concentration of
represents concentration of ![[NH_{3}]_{0}](/tpl/images/0641/7445/b342c.png) is initial concentration of
is initial concentration of ![[NH_{3}]=(-0.0038M.s^{-1}\times 20s)+0.333M](/tpl/images/0641/7445/bbef3.png)
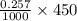 mol = 0.11565 mol
mol = 0.11565 mol

