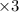Balance the redox reaction below using the half-reaction method. Sn2+(aq) + In(s)Sn(s) + In3+(aq) (a) To show your method, write the balanced half reactions below. Use the smallest integer coefficients possible and show electrons as e- . If a box is not needed, leave it blank. (Coefficients of 1 are not needed). Oxidation half-reaction: + + Reduction half-reaction: + + (b) To show your balanced equation, enter an integer in each of the boxes. If the integer is "1," do enter it even though you would normally not show that in the equation. Use the smallest integer coefficients possible. Sn2+(aq) + In(s) Sn(s) + In3+(aq)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
Balance the redox reaction below using the half-reaction method. Sn2+(aq) + In(s)Sn(s) + In3+(aq) (a...
Questions


Mathematics, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00

Business, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00

Mathematics, 15.02.2021 01:00


Social Studies, 15.02.2021 01:00




Computers and Technology, 15.02.2021 01:00


History, 15.02.2021 01:00



Mathematics, 15.02.2021 01:00

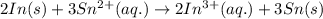
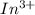 .
. 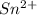 consume electrons and reduced to Sn.
consume electrons and reduced to Sn.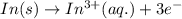 ]
]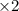
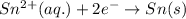 ]
]