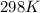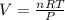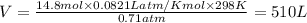Butane and oxygen were allowed to completely react at 540 torr and 298 K. After
the exothermic...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3


Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
Questions



Physics, 27.09.2020 03:01

Mathematics, 27.09.2020 03:01

Mathematics, 27.09.2020 03:01



History, 27.09.2020 03:01



English, 27.09.2020 03:01

Mathematics, 27.09.2020 03:01


Mathematics, 27.09.2020 03:01

Mathematics, 27.09.2020 03:01

Mathematics, 27.09.2020 03:01

Mathematics, 27.09.2020 03:01

Mathematics, 27.09.2020 03:01

Biology, 27.09.2020 03:01

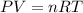
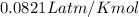
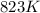
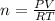
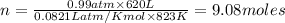
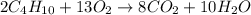
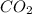 are produced by = 13 moles of oxygen
are produced by = 13 moles of oxygen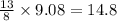 moles of oxygen
moles of oxygen