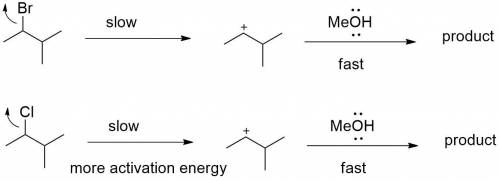
Chemistry, 05.05.2020 20:35 jacobp0712
Explain how the following changes would affect the rate of the reaction of 2-bromo-3-methylbutane with methanol: Part A The alkyl halide is changed to 2-chloro-3-methylbutane. The alkyl halide is changed to 2-chloro-3-methylbutane. The reaction will be slower because the leaving group will be poorer. The reaction will be faster because the leaving group will be better. The reaction will be slower because the leaving group will be better. The rate of the reaction does not change.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3


Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

You know the right answer?
Explain how the following changes would affect the rate of the reaction of 2-bromo-3-methylbutane wi...
Questions


Mathematics, 06.11.2020 04:00

Mathematics, 06.11.2020 04:00

Social Studies, 06.11.2020 04:00


Mathematics, 06.11.2020 04:00

History, 06.11.2020 04:00

Mathematics, 06.11.2020 04:00


World Languages, 06.11.2020 04:00

Physics, 06.11.2020 04:00

Mathematics, 06.11.2020 04:00

Mathematics, 06.11.2020 04:00

English, 06.11.2020 04:00

Physics, 06.11.2020 04:00

Geography, 06.11.2020 04:00


Mathematics, 06.11.2020 04:00

Mathematics, 06.11.2020 04:00

Mathematics, 06.11.2020 04:00

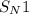 reaction.
reaction.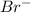 is a better leaving group than
is a better leaving group than 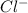 due to higher polarizability of
due to higher polarizability of