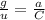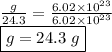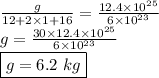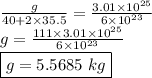Calculate the mass in grams of each of the following substances. 6.02 x 10²³ atoms of mg
...

Chemistry, 12.10.2019 20:30 deannajd03
Calculate the mass in grams of each of the following substances. 6.02 x 10²³ atoms of mg
calculate the mass in grams of each of the following substances.
12.4 x 10¹⁵ molecules of ch₂o
calculate the mass in grams of each of the following substances.
3.01 x 10²³ formula units of cacl₂

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

You know the right answer?
Questions








Mathematics, 24.06.2019 01:30



English, 24.06.2019 01:30





English, 24.06.2019 01:30


Mathematics, 24.06.2019 01:30


Mathematics, 24.06.2019 01:30