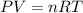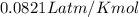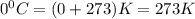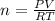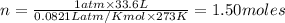Chemistry, 05.05.2020 21:29 milkshakegrande101
How many moles of oxygen (O2) are present in 33.6 L of the gas at 1 atm and 0°C?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
How many moles of oxygen (O2) are present in 33.6 L of the gas at 1 atm and 0°C?...
Questions

Mathematics, 17.12.2020 01:00


Mathematics, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00


History, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00

History, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00






Mathematics, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00



Mathematics, 17.12.2020 01:00