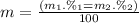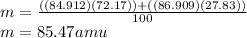Chemistry, 27.08.2019 07:00 Jerrikasmith28
Given that rubidium has two isotopes, 85rb and 87rb. calculate the average atomic mass of rubidium.
note that 85rb has an atomic mass of 84.912 amu and occurs at an abundance of 72.17% while 87rb has an atomic mass of 86.909 amu and occurs at an abundance of 27.83%.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1


You know the right answer?
Given that rubidium has two isotopes, 85rb and 87rb. calculate the average atomic mass of rubidium.<...
Questions


Geography, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

History, 07.10.2020 14:01


Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01


Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

English, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01



Mathematics, 07.10.2020 14:01


Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01