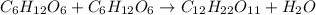Consider the following reaction.
c6h12o6+c6h12o6 -- c12h22o11
a. the equat...

Chemistry, 12.01.2020 01:31 mikayleighb2019
Consider the following reaction.
c6h12o6+c6h12o6 -- c12h22o11
a. the equation is not balanced; it is missing a molecule of water. write it in on the correct side of the equation.
b. what kind of reaction is this?
c. is c6h12o6 (glucose) a monomer, or a polymer?
d. to summarize, when two monomers are joined, a molecule of is always removed.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2


Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Questions







Computers and Technology, 20.03.2020 11:11




Biology, 20.03.2020 11:11







Social Studies, 20.03.2020 11:12

Mathematics, 20.03.2020 11:12