
Chemistry, 06.05.2020 02:31 cefindley14
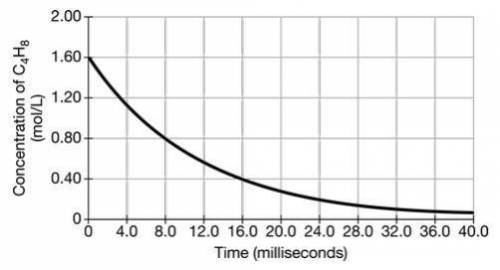
(i) Based on the graph, determine the order of the decomposition reaction of cyclobutane at 1270 K. Justify your answer.
(ii) Calculate the time, in milliseconds, that it would take for 99 percent of the original cyclobutane at 1270 K to decompose.
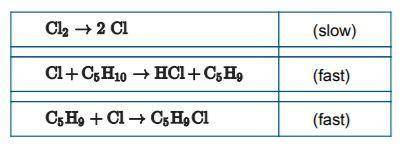
Another alkane with a ring structure is cyclopentane, C5H10(g). Cyclopentane reacts with
chlorine, Cl2(g) , to produce C2H9Cl(g) and HCl(g) . Following is a representation of a proposed mechanism for the reaction.
*kinetics table attached
(d) Write a rate law for the reaction that is consistent with the mechanism. Justify your answer.
(e) A student claims that, Cl2(g), is a catalyst in the reaction. Explain why the student’s claim is
false.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
(i) Based on the graph, determine the order of the decomposition reaction of cyclobutane at 1270 K....
Questions

English, 14.04.2021 23:00


History, 14.04.2021 23:00

Mathematics, 14.04.2021 23:00

Mathematics, 14.04.2021 23:00








Mathematics, 14.04.2021 23:00

Mathematics, 14.04.2021 23:00

Mathematics, 14.04.2021 23:00

Computers and Technology, 14.04.2021 23:00




Arts, 14.04.2021 23:00



