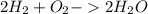Chemistry, 06.05.2020 17:00 bixbylily95
How many moles of water can be produced with 4.3 moles of H2 and 5.6 moles of O2? Which reactant is limiting ? How many moles of the excess will be left after the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3


Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
How many moles of water can be produced with 4.3 moles of H2 and 5.6 moles of O2? Which reactant is...
Questions

History, 14.06.2020 08:57


World Languages, 14.06.2020 08:57

Mathematics, 14.06.2020 08:57

Mathematics, 14.06.2020 08:57

Mathematics, 14.06.2020 08:57

Mathematics, 14.06.2020 08:57


Mathematics, 14.06.2020 08:57


Mathematics, 14.06.2020 08:57