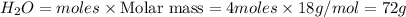What mass of H₂O is formed when excess H₂ reacts with 64 g of O₂
2(H2)+(O2)>2(H2O)
18...

Chemistry, 06.05.2020 18:02 felipe9086
What mass of H₂O is formed when excess H₂ reacts with 64 g of O₂
2(H2)+(O2)>2(H2O)
18g
36g
72g
144g

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
Questions


Social Studies, 16.09.2019 17:00

Mathematics, 16.09.2019 17:00

Chemistry, 16.09.2019 17:00

Geography, 16.09.2019 17:00


Mathematics, 16.09.2019 17:00

History, 16.09.2019 17:00


Mathematics, 16.09.2019 17:00


Social Studies, 16.09.2019 17:00

Mathematics, 16.09.2019 17:00




History, 16.09.2019 17:00

Biology, 16.09.2019 17:00

Advanced Placement (AP), 16.09.2019 17:00

Mathematics, 16.09.2019 17:00

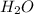 is formed.
is formed.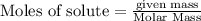
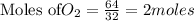
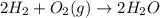
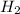 is the excess reagent,
is the excess reagent, 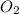 is the limiting reagent as it limits the formation of product.
is the limiting reagent as it limits the formation of product.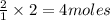 of
of