
Chemistry, 06.05.2020 18:59 madinaxoxo605
The [OH-] of an aqueous solution is 6.4 x 10-5 M. What is the [H3O+]?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
The [OH-] of an aqueous solution is 6.4 x 10-5 M. What is the [H3O+]?...
Questions



Mathematics, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

Chemistry, 23.02.2021 21:00

English, 23.02.2021 21:00






Mathematics, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00


Spanish, 23.02.2021 21:00

History, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

Mathematics, 23.02.2021 21:00

English, 23.02.2021 21:00

![[H_3O^+]](/tpl/images/0649/0280/6cfd2.png) is
is 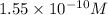
![pOH=-\log [OH^-]](/tpl/images/0649/0280/1fac1.png)
![pOH=-\log[6.4\times 10^{-5}]](/tpl/images/0649/0280/05710.png)
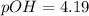
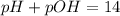
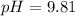
![9.81=-\log [H_3O^+]](/tpl/images/0649/0280/0025f.png)
![[H_3O^+]=1.55\times 10^{-10}](/tpl/images/0649/0280/427d6.png)



