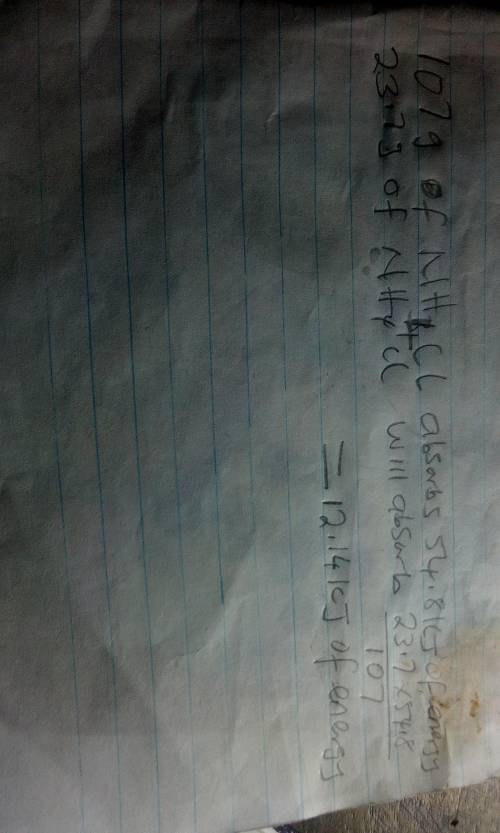Chemistry, 03.02.2020 20:45 PrincessIndia
The energy diagram shown represents the chemical reaction between solid ammonium chloride and solid barium hydroxide octahydrate:
2nh4cl(s) + ba(oh)2 x 8h2o(> 2nh3(aq)+bacl2(aq)+10h2o(1)
the (triangle then an h) for this reaction is 54.8 kj. how much energy would be absorbed if 23.7 g of nh4cl reacts?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
The energy diagram shown represents the chemical reaction between solid ammonium chloride and solid...
Questions

Geography, 03.05.2021 05:50


Mathematics, 03.05.2021 05:50

Mathematics, 03.05.2021 05:50

English, 03.05.2021 05:50


Mathematics, 03.05.2021 05:50

Mathematics, 03.05.2021 05:50

History, 03.05.2021 05:50





Mathematics, 03.05.2021 05:50

Mathematics, 03.05.2021 05:50

Health, 03.05.2021 05:50

Mathematics, 03.05.2021 05:50



English, 03.05.2021 05:50