
Chemistry, 07.05.2020 02:00 linnybear300
Enter your answer in the provided box. Lead(II) nitrate is added slowly to a solution that is 0.0400 M in Cl− ions. Calculate the concentration of Pb2+ ions (in mol / L) required to initiate the precipitation of PbCl2.
(Ksp for PbCl2 is 2.40 × 10−4.)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Enter your answer in the provided box. Lead(II) nitrate is added slowly to a solution that is 0.0400...
Questions



Mathematics, 14.01.2021 09:20


Mathematics, 14.01.2021 09:20


English, 14.01.2021 09:20

Mathematics, 14.01.2021 09:20

Biology, 14.01.2021 09:20

Mathematics, 14.01.2021 09:20

Mathematics, 14.01.2021 09:20





Mathematics, 14.01.2021 09:20


Mathematics, 14.01.2021 09:20

Mathematics, 14.01.2021 09:20

Mathematics, 14.01.2021 09:20

![[Pb^{2+}] } = 0.15 mol/L](/tpl/images/0651/0478/c6ffa.png)
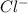 is
is 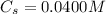

![K_{sp} = [Pb^{2+}][Cl^{-}]^2](/tpl/images/0651/0478/939e9.png)
![2.4 0 * 10^{-4} = 0.0400^2 * [Pb^{2+}]](/tpl/images/0651/0478/b2e7f.png)
![[Pb^{2+}] } = \frac{2.4 0 * 10^{-4} }{0.0400^2 }](/tpl/images/0651/0478/f08bf.png)


