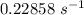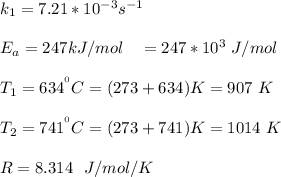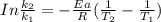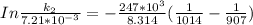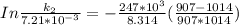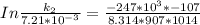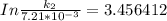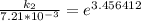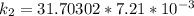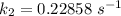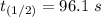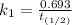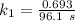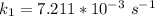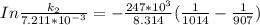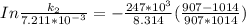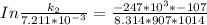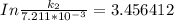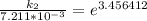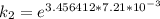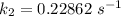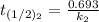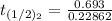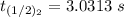The rate constant of the elementary reaction C2H5CN(g) → CH2CHCN(g) + H2(g) is k = 7.21×10-3 s-1 at 634 °C, and the reaction has an activation energy of 247 kJ/mol. (a) Compute the rate constant of the reaction at a temperature of 741 °C. s-1 (b) At a temperature of 634 °C, 96.1 s is required for half of the C2H5CN originally present to be consume. How long will it take to consume half of the reactant if an identical experiment is performed at 741 °C? (Enter numbers as numbers, no units. For example, 300 minutes would be 300. For letters, enter A, B, or C. Enter numbers in scientific notation using e# format. For example 1.43×10-4 would be 1.43e-4.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1


Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
The rate constant of the elementary reaction C2H5CN(g) → CH2CHCN(g) + H2(g) is k = 7.21×10-3 s-1 at...
Questions

Mathematics, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01


Computers and Technology, 03.09.2020 23:01


Mathematics, 03.09.2020 23:01




Mathematics, 03.09.2020 23:01

Mathematics, 03.09.2020 23:01



Mathematics, 03.09.2020 23:01




Mathematics, 03.09.2020 23:01

Social Studies, 03.09.2020 23:01