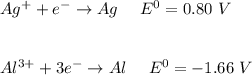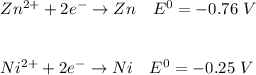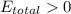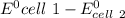Chemistry, 07.05.2020 04:59 kristieroth1
Consider two cells, the first with Al and Ag electrodes and the second with Zn and Ni electrodes, each in 1.00 M solutions of their ions. If connected as voltaic cells in series, which two metals are plated, and what is the total potential, E ∘ ? Which two metals are plated?
a. Al ( s )
b. Ag ( s )
c. Zn ( s )
d. Ni ( s )
E ∘ =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 09:30
How many moles of na2s2o3 are needed to react with 0.12mol of cl2? show work.
Answers: 1

You know the right answer?
Consider two cells, the first with Al and Ag electrodes and the second with Zn and Ni electrodes, ea...
Questions





Mathematics, 11.12.2019 21:31



Mathematics, 11.12.2019 21:31




History, 11.12.2019 21:31

Spanish, 11.12.2019 21:31

Mathematics, 11.12.2019 21:31

Mathematics, 11.12.2019 21:31

Mathematics, 11.12.2019 21:31

Mathematics, 11.12.2019 21:31


Mathematics, 11.12.2019 21:31

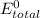 = 2.97 V
= 2.97 V