Chemistry, 07.05.2020 04:57 HotWheels162000
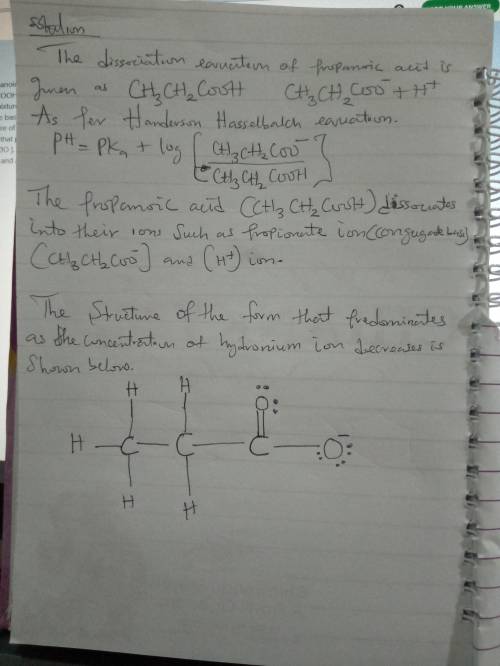
The pKa of propanoic acid (propionic acid), CH3CH2COOH, is 4.87. Consider an equilibrium mixture of propanoic acid and its conjugate base with a pH of 4.87. Draw the structure of the form (acid or conjugate base) that predominates after a decrease in [H3O ]. Include all hydrogen atoms and any appropriate formal charges.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
The pKa of propanoic acid (propionic acid), CH3CH2COOH, is 4.87. Consider an equilibrium mixture of...
Questions

Mathematics, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50



Biology, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50


History, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50





Spanish, 18.11.2020 21:50


Computers and Technology, 18.11.2020 21:50

Mathematics, 18.11.2020 21:50


Mathematics, 18.11.2020 21:50

English, 18.11.2020 21:50