
The cell potential for the cell Zn(s) + 2H+(? M) LaTeX: \longrightarrow⟶ Zn2+(1.3 M) + H2(g) (8 atm) is observed to be 0.68 V. What is the pH in the H+/H2 half-cell? Reduction potential for H2(g)/H+(aq) is 0.00 V, for Zn(s)/Zn2+(aq) is -0.76 V. Enter number to 2 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1


Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
The cell potential for the cell Zn(s) + 2H+(? M) LaTeX: \longrightarrow⟶ Zn2+(1.3 M) + H2(g) (8 atm)...
Questions



Mathematics, 02.06.2021 17:50




Mathematics, 02.06.2021 17:50


Mathematics, 02.06.2021 17:50

Mathematics, 02.06.2021 17:50

Geography, 02.06.2021 17:50


English, 02.06.2021 17:50



Mathematics, 02.06.2021 17:50

English, 02.06.2021 17:50

Mathematics, 02.06.2021 17:50

Mathematics, 02.06.2021 17:50

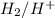 half cell is 0.84.
half cell is 0.84.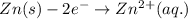
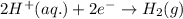
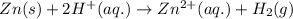
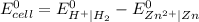 = (0.00 V) + (0.76 V) = 0.76 V
= (0.00 V) + (0.76 V) = 0.76 V![E_{cell}=E_{cell}^{0}-\frac{0.0592}{n}log\frac{[Zn^{2+}].P_{H_{2}}}{[H^{+}]^{2}}](/tpl/images/0651/7884/0ba03.png)
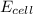 is cell potential, n is number of electron exchanged,
is cell potential, n is number of electron exchanged, 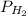 is pressure of
is pressure of 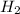 in atm and species under third bracket represent molarity of the respective species.
in atm and species under third bracket represent molarity of the respective species.![0.68V=0.76V-\frac{0.0592}{2}log\frac{(1.3M)\times (8atm)}{[H^{+}]^{2}}V](/tpl/images/0651/7884/ff1b4.png)

![[H^{+}]=0.1436M](/tpl/images/0651/7884/722f7.png)
![-log[H^{+}]](/tpl/images/0651/7884/1d5a1.png) = -log(0.1436) = 0.84
= -log(0.1436) = 0.84

