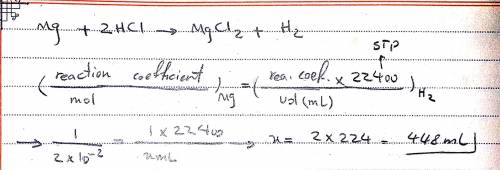Magnesium reacts with hydrochloric acid (HCl) as follows. How many milliliters of hydrogen gas are produced by the reaction 0.020 mol of magnesium with excess of hydrochloric acid at STP? Make sure to use the correct number of significant digits. 0.448 mL 0.45 mL 448 mL 450 mL

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2


Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
Magnesium reacts with hydrochloric acid (HCl) as follows. How many milliliters of hydrogen gas are p...
Questions

English, 23.11.2020 16:40

Mathematics, 23.11.2020 16:40

English, 23.11.2020 16:40



Arts, 23.11.2020 16:40


Mathematics, 23.11.2020 16:40


History, 23.11.2020 16:40



Mathematics, 23.11.2020 16:40

Physics, 23.11.2020 16:40


Mathematics, 23.11.2020 16:40

Mathematics, 23.11.2020 16:40