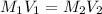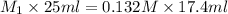Chemistry, 22.08.2019 13:00 carapiasebas
Calculate the concentration (in molarity) of naoh solution, if 25.0ml of the solution are needed to neutralize 17.4 ml of a 0.132m hcl solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
Calculate the concentration (in molarity) of naoh solution, if 25.0ml of the solution are needed to...
Questions

Mathematics, 02.02.2021 23:30


Mathematics, 02.02.2021 23:30

Mathematics, 02.02.2021 23:30


Mathematics, 02.02.2021 23:30


Mathematics, 02.02.2021 23:30




Mathematics, 02.02.2021 23:30

Mathematics, 02.02.2021 23:30

Biology, 02.02.2021 23:30


Business, 02.02.2021 23:30

Mathematics, 02.02.2021 23:30


Mathematics, 02.02.2021 23:30

Mathematics, 02.02.2021 23:30

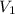 = 25 ml,
= 25 ml, 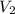 = 17.4 ml
= 17.4 ml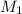 = ?,
= ?, 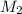 = 0.132 M
= 0.132 M