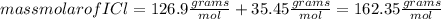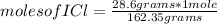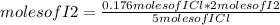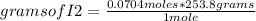Chemistry, 06.11.2019 07:31 shayravirgen30
Iodine chloride, icl, can be made by the following reaction between iodine, i2, potassium iodate, kio3, and hydrochloric acid.
2 i2 + kio3 + 6 hcl > 5 icl + kcl + 3 h2o
calculate how many grams of iodine are needed to prepare 28.6 grams of icl by this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
You know the right answer?
Iodine chloride, icl, can be made by the following reaction between iodine, i2, potassium iodate, ki...
Questions



Mathematics, 26.03.2021 19:00



Mathematics, 26.03.2021 19:00



Geography, 26.03.2021 19:00

History, 26.03.2021 19:00

Mathematics, 26.03.2021 19:00

Mathematics, 26.03.2021 19:00

English, 26.03.2021 19:00



Arts, 26.03.2021 19:00


Mathematics, 26.03.2021 19:00

Mathematics, 26.03.2021 19:00